An important step in the diagnosis of OHS is to confirm chronic (not acute) daytime hypercapnia by measuring room air arterial blood gases while resting in a sitting or supine position.
However, patients with OHS have an elevated serum bicarbonate level due to the metabolic compensation for the chronic respiratory acidosis. Therefore, serum bicarbonate level is a reasonable test to screen for hypercapnia because it is readily available, physiologically sensible, and less invasive than an arterial puncture to measure blood gases. It was recently shown that serum bicarbonate combined with the severity of obstructive sleep apnea (OSA) can be used as clinical predictors of OHS in patients with severe obesity and OSA.
Accordingly, arterial blood gases should be obtained to confirm the presence and severity of daytime hypercapnia in patients with obesity and sleep-disordered breathing that have hypoxemia on pulse oximetry during wakefulness or elevated serum bicarbonate level.
All patients with OHS suffer from sleep-disordered breathing.1 Therefore, the first step in the work up of a patient suspected of having OHS is to obtain an overnight in-laboratory attended polysomnogram or an attended respiratory polygraphy (without electroencephalography). In approximately 90% of patients with OHS the sleep-disordered breathing consists of obstructive sleep apnea (OSA). Due to this association the term "hypercapnic OSA" has been interchangeably used with OHS. The remaining 10% of patients with OHS have an AHI < 5. The sleep-disordered breathing in this subset of patients has been labeled as sleep hypoventilation and is defined as an increase in PaCO2 during sleep by 10 mm Hg above wakefulness or significant oxygen desaturation that is not explained by obstructive apneas or hypopneas.3
Although some clinicians favor the use of bi-level positive airway pressure versus continuous positive airway pressure (CPAP) for the treatment of OHS, two recent studies examined the efficacy of CPAP titration and long term therapy in patients with OHS.4, 5 Banerjee and colleagues compared the impact of CPAP titration on the sleep architecture, respiratory events, and nocturnal hypoxemia in 23 patients with OHS and 23 patients with eucapnic OSA matched for BMI, apnea-hypopnea index (AHI), and lung function. Both groups were extremely obese with severe sleep-disordered breathing and those with OHS had significant daytime hypercapnia. The protocol included a full night diagnostic polysomnogram followed by a full night of CPAP titration without supplemental oxygen therapy and CPAP was titrated to eliminate apneas, hypopneas, and any evidence of flow limitation. CPAP resolved sleep-disordered breathing and nocturnal hypoxemia in 57% of patients with OHS. The optimal CPAP pressure of 13.9±3.1 cm H2O was reached within one hour of sleep onset. CPAP was unable to resolve refractory hypoxemia in 43% of patients with OHS and these patients had a higher BMI, more severe nocturnal hypoxemia at baseline, and a higher residual AHI during the night of CPAP titration compared to those with successful titration.4 The fact that more than half of the patients with extreme but stable cases of OHS (based on BMI, AHI, and the level of daytime hypercapnia) were successfully titrated with CPAP--without requiring bi-level PAP or supplemental oxygen--suggests that the majority of patients with milder forms of OHS can be successfully titrated with CPAP as well. It is also important to point out that the findings of Banerjee et al do not apply to the subgroup of patients with OHS (10%) who do not have concomitant OSA.6
In a follow-up study, the same group of investigators performed a randomized controlled trial of CPAP vs. bi-level PAP therapy in patients with OHS without severe persistent nocturnal oxygen desaturation. At 3 month follow-up, both CPAP and bi-level PAP appeared to be equally effective in improving daytime hypercapnia and there was no difference with adherence between CPAP and bi-level PAP.5 The long-term treatment of choice in patients with persistent oxygen desaturation despite adequate CPAP titration remains to be elucidated and will most likely include a combination of bi-level PAP and supplemental oxygen.6
Therefore, there is no reason to empirically jump to bi-level PAP titration in patients with OHS. This patient may need daytime oxygen supplementation, but nocturnal oxygen supplementation alone is inadequate for treatment of OHS. Tracheostomy is successful in treating OHS, but should only be undertaken if positive airway pressure therapy is unsuccessful or the patient is unable to adhere to PAP therapy. Gastric bypass surgery would also be effective, but this patient's respiratory derangements should be corrected before he undergoes surgery to decrease perioperative complications.
OHS is defined as a combination of obesity (BMI >= 30kg/m2) and awake chronic hypercapnia (PaCO2 >= 45 mm Hg) accompanied by sleep-disordered breathing. It is important to recognize that OHS is a diagnosis of exclusion and should be distinguished from other conditions that are commonly associated with hypercapnia.1 These include advanced parenchymal lung disease, neuromuscular disease, chest wall disorders and severe hypothyroidism. Therefore, pulmonary function tests and chest imaging would be appropriate tests to assess for other secondary causes of hypoventilation, such as interstitial or obstructive lung disease. Assessment for severe hypothyroidism (thyroid stimulating hormone) is appropriate. Assessment for secondary erythrocytosis with a complete blood count is also appropriate, as it would alter the threshold for giving daytime oxygen therapy.
Multiple sleep latency test evaluation for objectively quantifying hypersomnolence and to exclude narcolepsy would only be clinically indicated if daytime hypersomnolence persists after successful treatment with positive airway pressure therapy.
The pulmonary function testing revealed a mild restrictive defect and the chest imaging was normal. An arterial blood gas was obtained on room air during an outpatient visit and revealed a pH of 7.35, PaCO2 of 56 mm Hg and PaO2 of 61 mm Hg.
The patient underwent a split night polysomnogram. The diagnostic portion of the study included 153 minutes of baseline sleep and demonstrated severe obstructive sleep apnea (OSA). The apnea-hypopnea index (AHI) was 105 events per hour, the lowest oxygen saturation was 65%, and the oxygen saturation was below 90% for 77 minutes or 50% of the pre-treatment portion of the study. OSA and the degree of oxygen desaturation were more severe in the supine position during which most respiratory events were apneas as opposed to hypopneas. The obstructive respiratory events also caused significant sleep fragmentation. Due to the severity of OSA, continuous positive airway pressure (CPAP) was started at 4 cm H2O and titrated to 10 cm H2O.
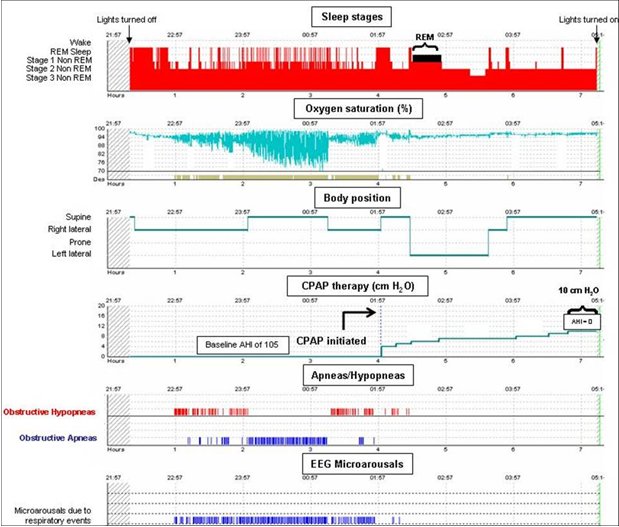
The improvement in hypercapnia and hypoxia is directly related to the daily dose of PAP therapy and maximum improvement in blood gases is achieved in as early as one month.3, 7 In a study of 75 ambulatory patients with OHS, the PaCO2 decreased by 1.8 mm Hg and the PaO2 increased by 3 mm Hg per hour of daily CPAP use. Moreover, patients who used CPAP for more than 4.5 hours per day experienced larger improvement in PaCO2 and PaO2 compared to less adherent patients (ΔPaCO2 7.7±5 vs. 2.4±4 mm Hg, p<0.001; ΔPaO2 9.2±11 vs. 1.8±9 mm Hg, p<0.001). Similarly, the need for daytime home oxygen therapy decreased from 30% to 6% in the adherent patients.8 Although changes in serum bicarbonate level and daytime room air pulse oximetry could be used as a less invasive measure of ventilation, a repeat measurement of arterial blood gases should be performed. If the PaCO2 fails to decline in that time, the most likely cause is non-adherence with PAP therapy. Therefore, it is imperative to objectively assess adherence with PAP therapy as patients frequently overestimate adherence. The improvement in hypercapnia in patients that are adherent with PAP therapy is neither universal nor complete because up to 25% of patients adherent with therapy fail to respond to CPAP. In this subset of patients, a repeat titration with bi-level PAP should be performed.
It is important to point out that OHS is often unrecognized and treatment is frequently delayed. A prospective study followed 47 patients with OHS after hospital discharge for 18 months. The mortality of patients with OHS was 23% vs. 9% in patients with a similar degree of obesity but without hypoventilation (hazards ratio 4.0) and most deaths occurred in the first three months after hospital discharge.9 Therefore, clinicians must maintain a high index of suspicion since early recognition and treatment may reduce the high burden of morbidity and mortality associated with this syndrome.




