Acute Hypoxemic Respiratory Failure: Ultrasound to the Rescue
Ziyad Al-Shathri
Pulmonary and Critical Care Fellow
George Washington University, Washington DC
A 62-year-old male was admitted with sepsis from a foot ulcer. His vitals were as follows: BP 110/60 mmHg, RR 25/min. Pulse 110/min and Temperature of 101F. He was started on antibiotics and given two liters isotonic saline bolus. His admission chest radiograph showed no infiltrates. On the next day the ulcer was debrided in the operating room and he received 5 liters of saline during the procedure. Immediately after the debridement he went into respiratory distress and was intubated for hypoxemic respiratory failure. On examination he had bilateral crackles on auscultation and his chest radiograph showed bilateral alveolar infiltrates. Bedside echocardiography showed normal left ventricular function with an estimated ejection fraction of 55%, normal diastolic filling pressures (E/e’ of 4) and an inferior vena cava diameter of 1.5 cm.
Lung sonography was performed. There was no pleural effusion on either side.
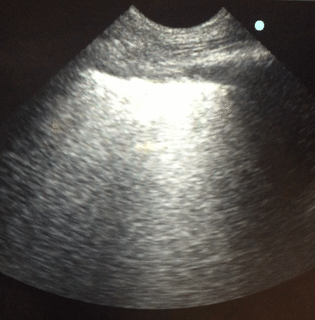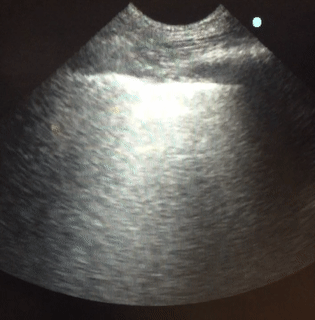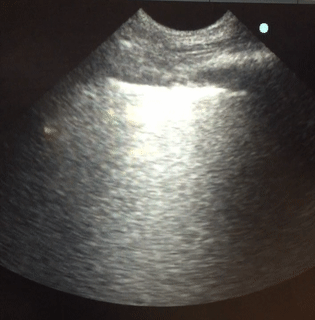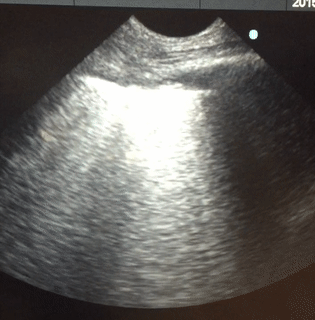
Q. The likely cause of acute hypoxemic respiratory failure in this case is:
-
Cardiogenic pulmonary edema
-
Acute Respiratory Distress Syndrome (ARDS)
-
COPD exacerbation
-
Pulmonary embolism
B
Discussion:
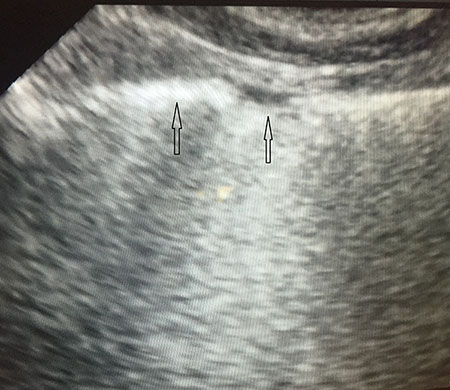
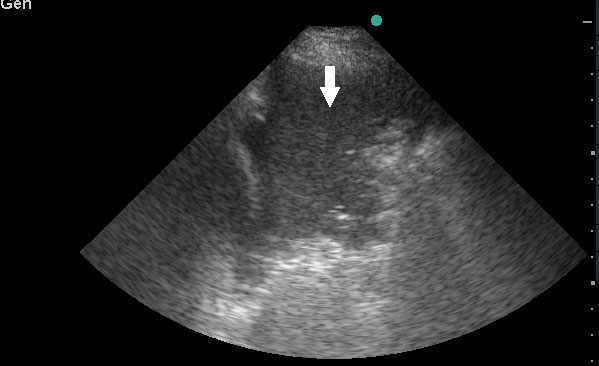
Lung sonography showed bilateral diffuse B lines (video), pleural sliding was preserved but the pleura was thickened (> 2mm) with areas of sub-pleural consolidation (figure 1). There were spared areas seen anteriorly on two points of examination (figure 1), pleural effusions were absent on either side and there was an area of consolidation seen anteriorly (figure 2).
The cause of acute hypoxemic respiratory failure (AHRF) can be difficult to ascertain in the early phase of the process. Sekiguchi et.al.1 identified cardiac and thoracic ultrasonography measurements to help identify the same during the early course of critical illness. In their study they identified the following variables that were significant in diagnosing CPE: presence of left-sided pleural effusion > 20 mm in the left posterolateral zone, degree of LV systolic dysfunction (moderate or severe dysfunction) and an IVC diameter > 23 mm. To facilitate clinical use of their prediction model they developed a simplified scoring system to help identify the likely cause of AHRF, with a score ≤ 3 consistent with a diagnosis of ARDS. In our patient the IVC was 15 mm, LV function was normal, there was no pleural effusion and he had a score of 1 based on the simplified scoring system in the study. Although the study has a reasonable diagnostic accuracy (sensitivity 77%, specificity 69%) it has limitations and it can be difficult to differentiate CPE for ARDS in certain patients. Those with pre-existing systolic dysfunction or pulmonary hypertension where the IVC can be dilated or patients with ARDS can have elevated pulmonary artery pressures which can cause the IVC to dilate. The significance of normal diastolic function with a low E/e’ ratio is that this indicates normal left ventricular filling pressures, which decreases the likelihood of cardiogenic pulmonary edema.2
Copetti et.al.3 in their study identified characteristic pleuro-pulmonary signs useful in the diagnosis of CPE and ARDS. As per their study the main signs that can be recognized by lung ultrasound in ARDS are as follows:
B-lines: In ARDS, B-lines should be bilateral and greater than 3 or more to confluence in a completely “white lung.” In the anterior lung fields, B-lines are not homogeneously distributed, whereas in the posterior lung fields B-lines are more compact and homogeneous, producing the image of a global white lung. While this finding is very sensitive for differentiating ARDS from cardiogenic pulmonary edema, it has poor specificity.
Spared areas: Areas of normal lung that are observed in at least one intercostal space, surrounded by areas of B-lines or white lung (usually in the anterior lung fields). This finding is very sensitive and specific for differentiating ARDS from cardiogenic pulmonary edema.
Consolidations: Areas of hyperechoic echotexture with punctiform elements or “hepatization,” with presence of static or dynamic air bronchograms. In ARDS, consolidations may be located in the posterior lung fields, especially at the bases. Consolidation is very specific and moderately sensitive for differentiating ARDS from cardiogenic pulmonary edema.
Pleural line abnormalities: Thickening is greater than 2 mm, and there is irregularity of the pleural line as well as evidence of small sub-pleural consolidations. In ARDS, the pleural line is always involved, and this leads to a reduction of lung sliding. In white lung areas, lung sliding might be absent and the pleural line may appear to move according to the heartbeat (lung pulse sign). The absence of lung sliding is very sensitive and specific for differentiating ARDS from cardiogenic pulmonary edema.
Pleural effusion: Anechoic and homogeneous pleural areas with no evidence of gas inside, limited by the diaphragm and pleura. If pleural effusion is likely to create a mechanical compression, the lower lung lobe can be visualized as collapsed and floating. Pleural effusion is less frequently observed in ARDS, although is often present (66.6% vs 95% in cardiogenic pulmonary edema). However, this is equally dependent on the primary cause of ARDS (e.g., pancreatitis, lower respiratory tract infection).
References:
-
Sekiguchi H, Schenck LA , Horie R et al. Critical Care Ultrasonography Differentiates ARDS, Pulmonary Edema, and Other Causes in the Early Course of Acute Hypoxemic Respiratory Failure. Chest 2015;148(4):912-918.
-
Nagueh S, Middleton K, Kopelen H et al. Doppler Tissue Imaging: A Noninvasive Technique for Evaluation of Left Ventricular Relaxation and Estimation of Filling Pressures. JACC 1997; 30(6):1527-1533.
-
Copetti R, Soldati G, Copetti P: Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound 2008; 6: pp. 16.



