An uncommon cause of orthopnea
Dustin L. Norton, MD1 and Jason R. Mock, MD PhD1
1Division of Pulmonary Diseases and Critical Care Medicine, Department of Medicine, University of North Carolina, Chapel Hill, North Carolina
Case
A 49-year-old man recently treated for pancreatitis presents with progressive dyspnea, orthopnea and new oxygen requirement. Pulmonary exam was remarkable for dullness to percussion and decreased breath sounds at the bases. There was inward movement of the abdominal wall with inspiration. ABG on room air revealed pH 7.43, pCO2 38, pO2 59. BNP was within normal limits and echocardiogram revealed a left ventricular ejection fraction of 55% without diastolic dysfunction.
Image 1
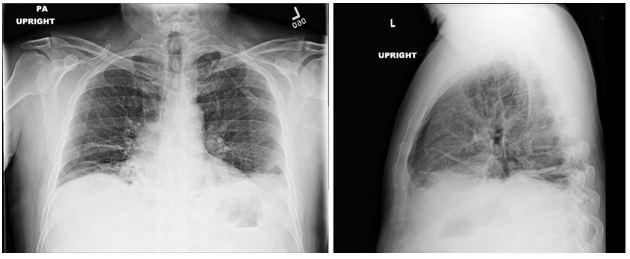
Image 2
Question
What is the most likely cause of the patient’s dyspnea and orthopnea?
- Congestive heart failure
- COPD
- Diaphragmatic paralysis
- Interstitial lung disease
Answers: C. Diaphragmatic paralysis
Discussion
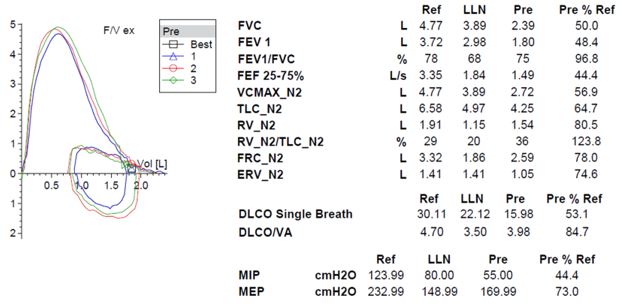
The patient’s symptoms, imaging, and pulmonary function testing (PFT) were consistent with diaphragmatic paralysis, with low lung volumes and bibasilar atelectasis. Fluoroscopy demonstrated minimal movement of both hemidiaphragms, consistent with bilateral diaphragmatic paralysis. Peripheral electromyography showed decreased amplitude of the right median sensory response and prolonged peak latency and decreased amplitude of the right radial sensory response. Motor units throughout the right arm were noted to be of high amplitude with long duration and reduced muscle recruitment. These findings were most consistent with mild to moderate, right-sided cervical radiculopathies. MRI of the spine was normal. Diagnosis was made of neuralgic amyotrophy with involvement of the bilateral phrenic nerves.
Answer A is incorrect as the patient had a normal echocardiogram and BNP. Answer B is incorrect as there was no obstruction on spirometry. Answer D is incorrect as there is no evidence of interstitial lung disease (ILD) on his chest x-ray nor crackles on exam to suggest an interstitial process. Additionally, an elevated RV to TLC ratio would argue against an ILD.
There are several causes of diaphragmatic paralysis (DP), including trauma, surgery, radiation, cervical manipulation, central nervous system lesions (amyotrophic lateral sclerosis, multiple sclerosis, poliomyelitis), peripheral nervous system lesions (brachial plexus neuropathy, Guillain-Barre, chronic inflammatory demyelinating polyneuropathy, post-viral or idiopathic phrenic neuropathy), and myopathies (1). Neuralgic amyotrophy is an inflammatory neuropathy that generally affects the brachial plexus with occasional involvement of the phrenic nerve(s). It is often characterized by sudden onset of severe neuropathic pain involving the shoulders and upper extremities followed by weakness and/or sensory loss in the affected areas. There are both hereditary and idiopathic forms of neuralgic amyotrophy with relapse rates of around 25% and 75% for idiopathic and hereditary forms, respectively (2). There is no treatment of neuralgic amyotrophy, though patients generally experience resolution of symptoms over months to years (3).
DP can be unilateral or bilateral and range from asymptomatic to having severe orthopnea and resting dyspnea with chronic respiratory failure. DP is characterized by restrictive physiology on PFTs with forced vital capacity (FVC) generally less than 50% of predicted in bilateral diaphragmatic paralysis (BDP). Supine spirometry can be helpful in diagnosis and generally shows a 10-30% decline in FVC in patients with unilateral diaphragmatic paralysis (UDP) and as much as a 50% decline in BPD. While functional residual capacity (FRC) is often mildly decreased, this is contrary to the significant reduction seen in ILD. DLCO is also frequently reduced, though this reduction is due to reduced alveolar volume and should improve when this correction is factored in, contrary to the fixed reduction seen in ILD (4). Diaphragmatic fluoroscopy, or sniff test, shows paradoxical upward movement of the affected diaphragm with inspiration. Maximal inspiratory force (MIP) is relatively preserved in UDP (generally >60%) but is often severely reduced (<30%) in BDP (5). Physical exam may show paradoxical inward movement of the abdomen with inspiration due to negative pleural pressures generated by accessory muscles pulling the diaphragm superiorly, though this is generally only seen in BDP. Chest imaging often shows elevation of the affected diaphragm(s) with associated lower lobe atelectasis (6). More recently, ultrasound has been shown to be an effective and noninvasive way to accurately assess diaphragm function (7). Treatment of DP is often supportive, though patients with symptomatic DP may benefit from diaphragm plication to limit upward movement of the affected diaphragm with inspiration and nocturnal noninvasive ventilation if hypercapnia develops (8). Phrenic nerve pacing can be considered in patients who require mechanical ventilation, particularly in patients with BPD secondary to cervical spine injuries (9).
Our patient had severe symptoms initially with resting hypoxemia. He was started on nocturnal noninvasive positive pressure ventilation (NIPPV) and supplemental oxygen. He noted slow improvement in his symptoms over time. Sniff test was repeated at 10 months and showed improved diaphragm excursion. Repeat PFT at 12 months showed improvement in FVC to 77% of predicted. He has since been weaned from both NIPPV as well as supplemental oxygen.
References:
-
Billings ME, Aitken ML, Benditt JO. Bilateral diaphragm paralysis: a challenging diagnosis. Respir Care 2008; 53(10): 1368-71.
-
van Alfen N. The neuralgic amyotrophy consultation. J Neurol 2007; 254(6):695-704.
-
van Alfen N, van Engelen BG, Hughes RA. Treatment for idiopathic and hereditary neuralgic amyotrophy (brachial neuritis). Cochrane Database Syst Rev 2009(3): CD006976.
-
Dube BP, Dres M. Diaphragm Dysfunction: Diagnostic Approaches and Management Strategies. J Clin Med 2016; 5(12). pii: E113.
-
Koo P, Oyieng'o DO, Gartman EJ, et. al. The Maximal Expiratory-to-Inspiratory Pressure Ratio and Supine Vital Capacity as Screening Tests for Diaphragm Dysfunction. Lung 2017; 195(1): 29-35.
-
McCool FD, Tzelepis GE. Dysfunction of the diaphragm. N Engl J Med 2012; 366(10): 932-42.
-
Sferrazza Papa GF, Pellegrino GM, Di Marco F, et. al. A Review of the Ultrasound Assessment of Diaphragmatic Function in Clinical Practice. Respiration 2016; 91(5): 403-11.
-
Welvaart WN, Jak PM, van de Veerdonk MC, et. al. Effects of diaphragm plication on pulmonary function and cardiopulmonary exercise parameters. Eur J Cardiothorac Surg 2013; 44(4): 643-7.
-
DiMarco AF. Diaphragm Pacing. Clin Chest Med 2018; 39(2): 459-71.



